Pipeline overview
SymBio continues to introduce new drug development candidates from bio-ventures and pharmaceutical companies around the world that have high medical needs even if the market is relatively small, and that have an established POC (Proof of Concept) in humans. We aim to commercialize it in a short period of time.
Going forward, SymBio will continue to aggressively expand indications (additional indications) for products under development, while continuously in-licensing and developing development candidates with a high probability of commercialization, thereby building a strong pipeline portfolio.
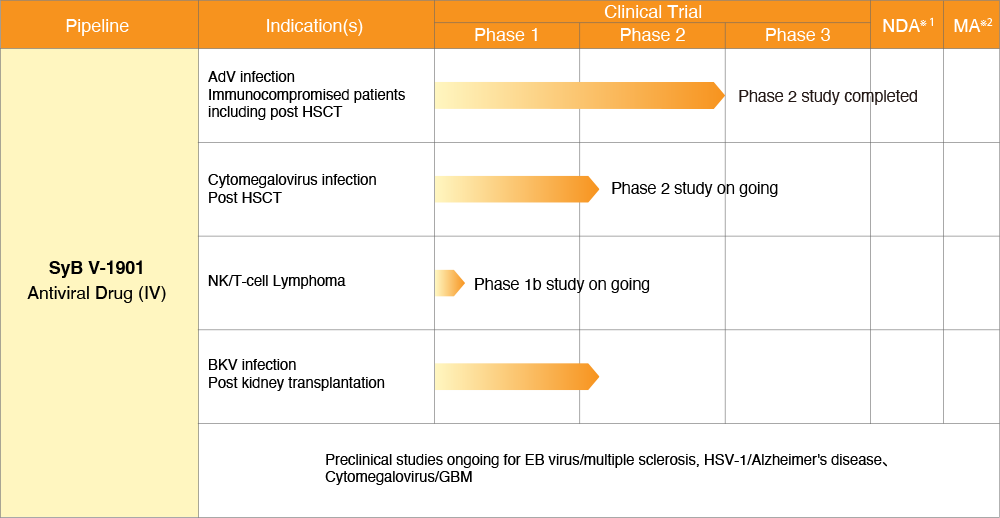
brincidofovir
TREAKISYM_
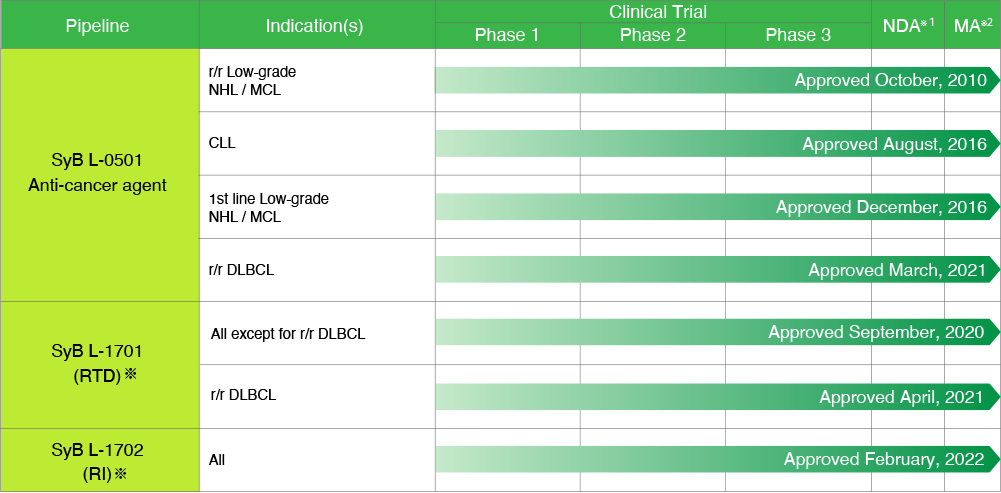
*On September 20, 2017, SymBio acquired the license rights for bendamustine liquid formulation (RTD formulation, RI administration) from Eagle Pharmaceuticals, Inc. (New Jersey, USA). SymBio will start selling RTD formulations in January 2021, and will gradually introduce RI administration to the market.
RTD: Ready To Dilute, RI: Rapid Infusion
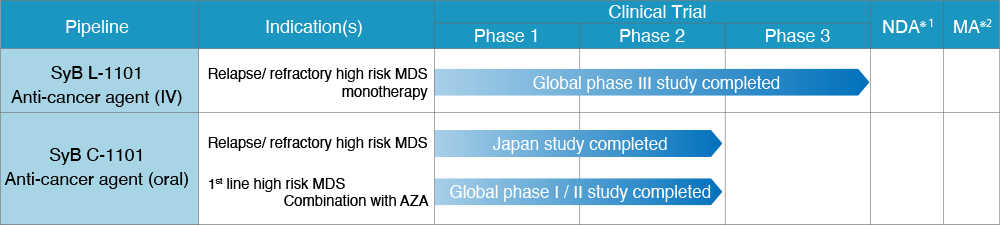
rigosertib sodium