Proprietary drug discovery business
[Outstanding new drug development capabilities and in-house sales and distribution capabilities]
SymBio Pharmaceuticals has obtained approval and launched sales of the anti-cancer agent Treakisym just five years after obtaining the license. Compared to the typical new drug development period of 9 to 16 years, this is an overwhelmingly fast pace, demonstrating the extremely high probability of success of our unique new drug development model. Here are seven advantages of this development model.
SymBio 's unique drug discovery business that handles everything from new drug development to approval, sales and distribution
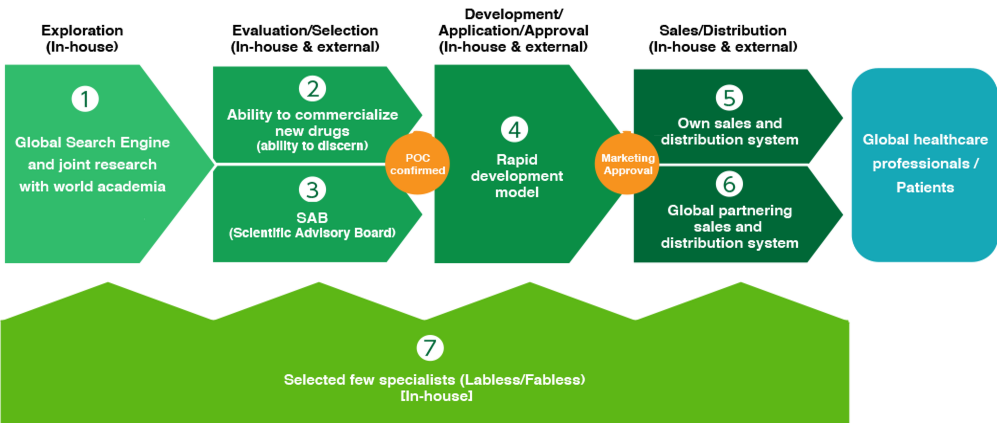
① Global Search Engine
SymBio has built a network directly connected to pharmaceutical companies, bio-ventures, and university research institutes around the world. We search for new drug candidates while constantly monitoring research around the world, and carefully select and introduce new drug candidates from this vast amount of information.
(2) Judgment ability based on abundant experience and achievements
SymBio's top management and development staff visit information on promising new drug candidates searched from around the world and hold repeated discussions, ascertaining the feasibility of commercialization based on our experience and achievements. increase. In general, the probability of approval for a new drug candidate that has entered clinical trials is approximately 1/13.
③ Scientific Advisory Board (SAB) where the world's brains gather
SymBio has established a "Scientific Advisory Board" (commonly known as "SAB") consisting of clinicians and basic scientists from around the world who have excellent achievements and experience. Organizing. SAB carefully evaluates promising new drug candidates after thoroughly discussing high medical needs, profitability, etc.
④ Speedy development model based on late-stage strategy
In general, new drug development requires several stages of basic research, preclinical studies, and clinical trials, and takes 9 to 16 years. On the other hand, SymBio does not conduct any basic research, and focus on the late stage, called “late stage,” where efficacy and safety have already been confirmed in humans. SymBio has introduced new drug candidates that are expected to be effective and safe in humans from such sources, and have significantly shortened the time to approval. This strategy has enabled new drug approvals within the shortest timeline of four to six years.
⑤ In-house sales and distribution system
In addition to our outstanding new drug development capabilities, another feature that supports our drug discovery business is our compact and highly specialized in-house sales and distribution system for TREAKISYM that we launched in December 2020.
○ Information provision and proposal ability by MRs specializing in TREAKISYM
The greatest feature of our sales organization is its high degree of specialization. "Hematology Experts" who have a strong relationship of trust with Hematology Expert specialists collaborate with blood cancer specialist MRs "Treakisym Manager" located nationwide. SymBio provides information and proposals based on the latest knowledge to doctors and medical professionals.
○ Nationwide distribution system for timely and reliable delivery
For smooth and stable supply, we have built a nationwide distribution system with two companies, the Suzuken Group and KYOSO MIRAI GROUP as sole agents. In addition, in collaboration with S.D.Collaboration, SymBio has two distribution bases in eastern Japan and western Japan, which provide prompt delivery to medical institutions nationwide under strict quality control.
⑥ Global partnering sales and distribution system
The global strategic product brincidofovir is expected to be used not only in viral infections, but also in various therapeutic areas such as hematology, cancer, and neurodegenerative disease, and is currently undergoing clinical development in countries around the world. In addition to our own sales and distribution system, we plan to develop a sales and distribution system through global partnering that matches the characteristics of the regional market, according to the development status in the future.
⑦ Minority experts
SymBio Pharmaceuticals is a labless/fabless (no research or production facilities) company consisting of only a small number of elite specialists. SymBio has built a low-cost development system through collaboration with external companies and practice highly mobile management.